Skin is the first line of protection in human physiology to avoid damaging from environmental stressors. Skin ageing is a process affected by both genetic and environmental factors while the cumulation of environmental factors can accelerate skin ageing or even lead to skin diseases. Human skin is exposed to environmental insults on a daily basis, particularly UV radiation, which triggers a range of molecular responses. Other stressors can include chemicals, tobacco smoke, fossil fuel pollutants, seasonal climate changes, and even internal in the form of cortisol from psychological stress [1].
In the skin, the dermal matrix contains functional proteins such as collagen, elastin, and proteoglycans which is responsible for conferring strength and resiliency of the skin. Skin ageing associated with dermal matrix alterations and atrophy can be caused by senescence of dermal cells such as fibroblasts, and decreased synthesis and accelerated breakdown of dermal collagen fibres [2].
Molecular mechanism of skin ageing
As shown in Figure 1, due to genetic ageing and cumulative exposure to UV radiation, the increase of reactive oxygen species (ROS) and the decrease of DNA repair capacity result in oxidative DNA damage with high levels of 8-oxo-dG lesions. Reduced NAD+ biosynthesis and/or NAD+ consumption induce cellular energy depletion that leads to decreased DNA repair activity and PARP-1 inhibition. Both NAD+ depletion and DNA damage can induce cell senescence or apoptosis. Senescent cell accumulation in the tissue is associated with chronic inflammation. Replenishment of the NAD+ pool by the supplement of NAD+ precursors, e.g., NAM, counteracts the inflammatory responses and the suppression of immunity, and further enhances DNA repair to maintain genomic stability [3].
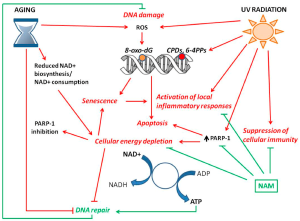
Figure 1. Mechanism of skin ageing by major intrinsic and extrinsic factors [3].
NAD+ homeostasis and skin photoaging
UV radiation causes skin photoaging, which can damage the skin cells by different approaches including DNA damage, reduction of DNA repair capacity, activation of local inflammatory responses and suppression of cellular immunity [4]. In an animal model, several histological changes were found in the skin, as shown in Figure 2 [5]. The epidermis of photodamaged skin is thicker than that of intrinsically aged skin, whereas the epidermis of severely photodamaged skin elicits epidermal atrophy [6]. Photoaged skin can also present more inflammatory cells [7]. Meanwhile, the most pronounced histological feature of photoaging is the disintegration of elastic fibres (solar elastosis), which leads to accumulation of amorphous, thickened, curled, and fragmented elastic fibres [8].
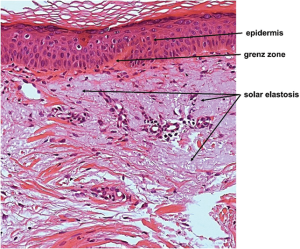
Figure 2. Histology of photoaged skin [5].
Recently, the NAD+ precursor, NAM, has been shown some of the significant acute and chronic damaging effects of UV exposure on skin [9-11]. It has been studied that nicotinamide can prevent UV-induced gene expression of the inflammatory mediators IL-6 and TNF [12] as well as DNA damage by enhancing the repair of cyclobutane pyrimidine dimers (CPDs) and 8-oxo-7,8-dihydro-2-deoxyguanosine [11]. Based on the biochemical pathways in vitro and in vivo studies, it is widely believed that one of NAM’s significant role in human skin is to rapidly incorporate and resupply cellular NAD+ pools, thereby facilitating and/or restoring efficient metabolism and ATP synthesis [13,14].
NAD+ homeostasis and chronic skin ageing
Genetically aged dermal fibroblasts also displayed reduced NADPH/NADP+ redox ratio and collagen secretion compared to those from young samples. In aged cells, the biosynthetic enzyme NAMPT expression decreases in several tissues during ageing, thus leading to NAD+ deficiency, reduced Sirt1 activity and, in turn, to cell senescence [15]. Direct analysis of NAD+ in human skin samples collected from the pelvic region shows that there is a significant NAD+ depletion in total pool content as a function of age [16]. More recently, a decrease in fluorescence emission from NADPH in the facial skin of older aged females compared to younger ones was noticed [17]. Relative to cellular bioenergetics, microarray analysis of human skin samples collected after NAM treatment has led to the mechanistic hypothesis that part of its protective effects involves protection of cellular metabolism [18], including both oxidative phosphorylation and glycolysis [19]
The integration of various research has shown that the central role of NAD+ is related to various mechanisms and processes for maintaining skin cell homeostasis. The restore and supplement of NAD+ may become a very important intervention method that can prevent skin ageing and restore it to a normal homeostatic state [20].
References:
- Burke KE, Wei H. Synergistic damage by UVA radiation and pollutants, Toxicol. Ind. Health 2009. 25: 219–224.
- Murakami H. et al. Importance of amino acid composition to improve skin collagen protein synthesis rates in UV-irradiated mice. Amino Acids, 2012. 42(6): 2481–2489.
- Fania L, Mazzanti C, Campione E, Candi E, Abeni D, Dellambra E. Role of Nicotinamide in Genomic Stability and Skin Cancer Chemoprevention. Int. J. Mol. Sci. 2019, 20: 5946.
- Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21: 16–29.
- Kim M, Park H J. Molecular Mechanisms of Skin Aging and Rejuvenation, Molecular Mechanisms of the Aging Process and Rejuvenation, Naofumi Shiomi, IntechOpen, 2016.
- Bhawan J, et al. Photoaging versus intrinsic aging: a morphologic assessment of facial skin. J Cutan Pathol, 1995. 22(2): 154–159.
- Bosset S, et al. Photoageing shows histological features of chronic skin inflammation without clinical and molecular abnormalities. Br J Dermatol, 2003. 149(4): 826–35.
- Tsuji T, Loss of dermal elastic tissue in solar elastosis. Arch Dermatol, 1980. 116(4): 474–475.
- Damian DL. Photoprotective effects of nicotinamide, Photochem. Photobiol. Sci. 2010. 9: 578–585.
- Surjana D, Damian DL. Nicotinamide in dermatology and photoprotection, Skinmed 2011. 9: 360–365.
- Surjana D, Halliday GM, Damian DL. Nicotinamide enhances repair of ultraviolet radiation-induced DNA damage in human keratinocytes and ex vivo skin, Carcinogenesis 2013. 34: 1144–1149.
- Monfrecola G, Gaudiello F, Cirillo T, Fabbrocini G, Balato A, Lembo S. Nicotinamide downregulates gene expression of interleukin-6, interleukin-10, monocyte chemoattractant protein-1, and tumour necrosis factor gene expression in HaCaT keratinocytes after ultraviolet B irradiation, Clin. Exp.Dermatol. 2013. 38: 185–188.
- Maiese K, Chong ZZ, Hou J, Shang YC, The vitamin nicotinamide: translating nutrition into clinical care, Molecules 2009, 14: 3446–3485.
- Stein LR, Imai S. The dynamic regulation of NAD metabolism in mitochondria, Trends Endocrinol. Metab. 2012. 23: 420–428.
- Aman Y, Qiu Y, Tao J, Fang EF. Therapeutic potential of boosting NAD+ in aging and age-related diseases. Transl. Med. Aging 2018, 2: 30–37.
- Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Ageassociated changes in oxidative stress and NAD+ metabolism in human tissue, PLoS One 2012. 7: e42357.
- Miyamoto K, Kudoh H. Quantification and visualization of cellular NAD(P)H in young and aged female facial skin with in vivo two-photon tomography, Br. J. Dermatol. 2013. 169: 25–31.
- Damian DL, Patterson CR, Stapelberg M, Park J, Barnetson RS, Halliday GM. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide, J. Invest. Dermatol. 2008. 128: 447–454.
- Park J, Halliday GM, Surjana D, Damian DL, Nicotinamide prevents ultraviolet radiation-induced cellular energy loss, Photochem. Photobiol. 2010. 86: 942–948.
- Oblong JE. The evolving role of the NAD+/nicotinamide metabolome in skin homeostasis, cellular bioenergetics, and aging. DNA Repair (Amst). 2014. 23:59-63.


